An aging population will bring colossal health, social, and economic challenges over the coming decades. As people live longer, staving off the physical decline and frailty that come with age has become a holy grail, with effective interventions projected to unlock significant societal and economic benefits. Estimates suggest that a slowdown in aging that increases life expectancy by one year alone is worth US$38 trillion. In a discovery published in Nature, a team of scientists from Duke-NUS Medical School in Singapore may have found a key to slow aging.
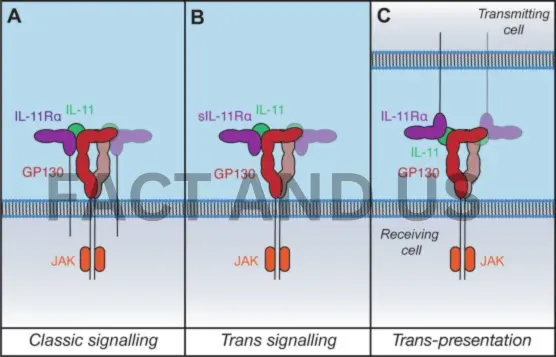
The team demonstrated in preclinical models that the protein interleukin-11 (IL-11) actively promotes aging and that giving an anti-IL-11 therapy not only counteracts the deleterious effects of aging but also increases lifespan. Their discovery has the potential to play a significant role in countries’ efforts to help their population live more years in good health.
In a recent study published in the journal Nature, a team of researchers used murine models and various pharmacological and genetic approaches to examine whether pro-inflammatory signaling involving interleukin (IL)-11, which activates signaling molecules such as extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin complex 1 (mTORC1), was negatively associated with health- and lifespan.
Contents
IL-11 leads to fat accumulation and muscle mass loss, two key hallmarks of aging
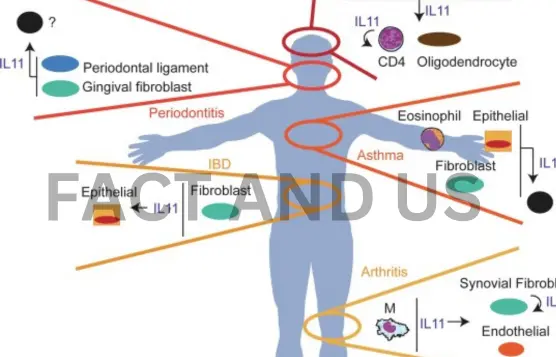
In preclinical studies, the team found that with age, organs expressed increasing levels of the IL-11 protein, which, in turn, promoted fat accumulation in the liver and abdomen, and reduced muscle mass and strength—two conditions that are hallmarks of human aging. According to the team, these results are the first in the world to demonstrate that IL-11 is a principal factor in aging.
First and co-corresponding author Assistant Professor Anissa Widjojo from Duke-NUS’ Cardiovascular and Metabolic Disorders Program, said, “This project started back in 2017 when a collaborator of ours sent us some tissue samples for another project. Out of curiosity, I ran some experiments to check for IL-11 levels. From the readings, we could clearly see that the levels of IL-11 increased with age and that’s when we got really excited.”
Anti-IL-11 therapy counteracts effects of aging
After establishing IL-11’s role in aging, the team demonstrated that by applying anti-IL-11 therapy in the same preclinical model, metabolism was improved, shifting from generating white fat to beneficial brown fat. Brown fat breaks down blood sugar and fat molecules to help maintain body temperature and burn calories. The researchers also observed improved muscle function and overall better health in their study, as well as an increased lifespan by up to 25 percent in both sexes.
Unlike other drugs known to inhibit specific pathways involved in aging, such as metformin and rapamycin, anti-IL-11 therapy blocks multiple major signaling mechanisms that become dysfunctional with age, offering protection against multimorbidity from cardiometabolic diseases, age-related loss of muscle mass and strength as well as frailty.
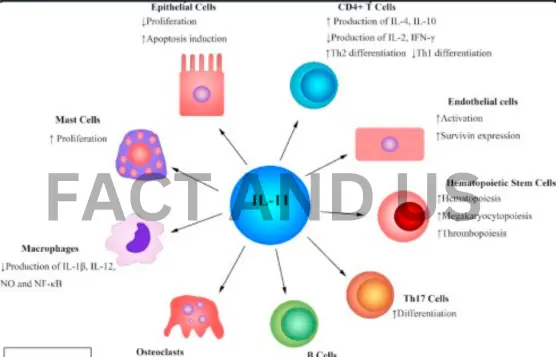
In addition to these externally observable changes, anti-IL-11 therapy also reduced the rate of telomere shortening and preserved mitochondria’s health and ability to generate energy. Senior author Tanoto Foundation Professor of Cardiovascular Medicine at the Sing Health Duke-NUS Academic Medical Center Stuart Cook, who is also with Duke-NUS’ Cardiovascular and Metabolic Disorders Program, said, “Our aim is that one day, anti-IL-11 therapy will be used as widely as possible, so that people the world over can lead healthier lives for longer. However, this is not easy, as approval pathways for drugs to treat aging are not well-defined, and raising funds to do clinical trials in this area is very challenging.”
Assessing the potential of the research, Professor Thomas Coffman, Dean of Duke-NUS, said, “Despite average life expectancy increasing markedly over recent decades, there’s a notable disparity between years lived and years of healthy living, free of disease. For rapidly aging societies like Singapore’s, this discovery could be transformative, enabling older adults to prolong healthy aging, reducing frailty and risk of falls while improving cardiometabolic health.”
The team’s previous research on IL-11’s role in the heart and kidney (published in Nature in 2017), liver (published in Gastroenterology in 2019) and lung (published in Science Translational Medicine in 2019) led to the development of an experimental anti-IL-11 therapy.
In this latest work, the Duke-NUS team collaborated with scientists from the National Heart Center Singapore; the MRC Laboratory of Medical Sciences in the UK; the Max Delbruck Center for Molecular Medicine in Germany; and the University of Melbourne in Australia.
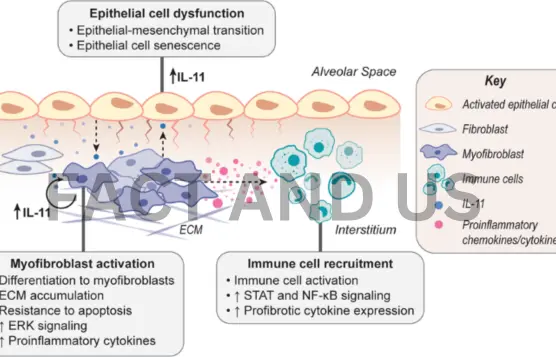
Background
Research suggests that hallmarks of aging such as inflammation, cellular senescence, and mitochondrial dysfunction are associated with perturbations in pathways associated with signaling molecules such as ERK, mTORC1, adenosine monophosphate-activated protein kinase (AMPK), and serine/threonine kinase 11 (STK11). Metabolic health in aged organisms is linked to the AMPK-mTORC1 axis, and the inhibition of mTOR in mice models has been found to extend the animal’s lifespan.
Studies in animal models such as fruit flies, yeast, and worms have explored lifespan extension. However, the findings from these studies cannot directly be applied to health span since lifespan extension cannot be equated with improved health span. Therefore, it is important to explore the impact of various interventions on lifespan and health span separately. In this respect, mice are suitable models as they exhibit aging pathologies similar to those of humans.
Furthermore, chronic sterile inflammation is a characteristic of a dysfunctional immune system and one of the crucial hallmarks of aging. Interventions targeting pro-inflammatory cytokines could have positive effects on health- and lifespan.
About the study
In the present study, the researchers hypothesized that IL-11, a pro-fibrotic and pro-inflammatory cytokine belonging to the IL-6 family of cytokines, could be involved in pathologies related to aging and lifespan reduction. They based this hypothesis on the role of IL-11 in activating the ERK-mTORC1 and Janus kinase-signal transducers and activators of transcription-3 (JAK-STAT3) pathways.
For this study, the researchers used mouse models and human hepatocyte cultures. Primary human hepatocytes were cultured and then stimulated with IL-11 for varying durations. The supernatants from these stimulated cells were then used for proximity extension assays using an inflammation panel consisting of 92 proteins.


Additionally, human cardiac fibroblasts treated with immunoglobulin G (IgG) or X209, the neutralizing antibody that targets the IL-11 receptor alpha subunit (IL11RA), were used for high throughput phenotyping assay. Serum-starved human cardiac fibroblasts were also used to measure the mitochondrial oxygen consumption rate and fatty acid oxidation rate.
Three strains of mice were used for the animal model experiments — mice with deleted interleukin 11 receptor subunit alpha 1 (IL11RA1) gene, mice with deleted IL11 gene, and mice with the gene for enhanced green fluorescent protein (EGFP) inserted into the IL11 gene. These mice were subjected to various treatments, such as IL-11 deletion and administration of anti-IL11 antibodies, and used to assess metabolic parameters, physiological traits, and lifespan. Lifespan and tumor progression were monitored after the mice were intraperitoneally injected with IgG or anti-IL-11 antibodies. The animals were also subjected to glucose and insulin tolerance tests, and echo magnetic resonance imaging was used to analyze their body composition.
Grip strength assessments were also conducted, and indirect and bomb calorimetry was performed to measure the body’s metabolic parameters and energy content from stool samples. Additionally, various assays, including colorimetry, were used to assess biomarkers such as cholesterol, collagen, and various interleukin levels and liver function parameters such as alanine transaminase and aspartate aminotransferase activities. The researchers also conducted quantitative polymerase chain reaction and sequencing of ribonucleic acid (RNA) extracted from the cells and immunoblotting using protein extracted from various tissues such as the liver, visceral gonadal white adipose tissue, and skeletal muscle. Histological and immunofluorescence-based examinations were also performed.
Results
The study found that in aging mice, the expression of IL-11 was upregulated in various types of cells and tissues and that the deletion of either the gene coding for IL-11 or the IL-11 receptor’s alpha 1 subunit protected the mice against metabolic decline, frailty, and multimorbidity as they aged.
Furthermore, administering antibodies against IL-11 in mice aged 75 weeks and older for both sexes for 25 weeks improved muscle function, boosted metabolism, lowered aging biomarkers’ levels, and reduced frailty. The deletion of the IL11 gene was found to extend the lifespan of the mice by an average of 24.9%, and treatment of 75-week-old mice with anti-IL-11 antibodies increased the median lifespan of male and female mice by 22.5% and 25%, respectively. Additionally, given that mortality in mice due to old age is often cancer-related, it was observed that IL-11 inhibition significantly lowered the incidence of age-related cancers and tumorigenesis.
Conclusions
To conclude, the results highlighted the detrimental role of the pro-inflammatory cytokine IL-11 in mammals’ health span and lifespan. The study found that anti-IL-11 antibodies improved metabolic parameters and muscle function and lowered cancer incidence in mouse models. These findings indicate that therapeutic targeting of IL-11 might be valuable in cancer therapy and treating fibrotic lung disease.
Stay connected with Fact and US for more such news.
